-
Dalton & Henry's Laws
In these two laws, we find the reason divers need to decompress, and the cause of shallow water blackout.
Dalton's Law
The total pressure exerted by a mixture of non-reactive gasses is equal to the sum of the partial pressures of the individual gasses.
Ptotal = P1 + P2 + P3 + ....
Henry's Law
The amount of gas that will dissolve into a liquid is directly porportional to the partial pressure of that gas in the surrounding medium.
Note: I will not go into the mathmatics on this one!
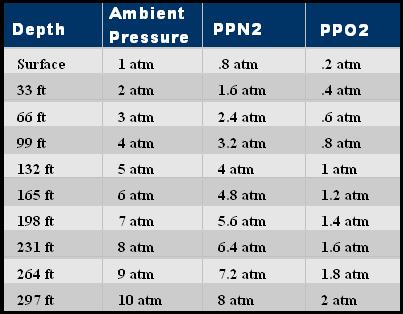
In the air that we breathe, the air is made up of various gasses, each of which adds together to form the 14.7 psi that we see at sea level. This is what is described by Dalton's Law. Each partial pressure of the gas equal to it's precentage in the mix.
Air
Nitrogen 78.084%
Oxygen 20.946%
Trace Gasses 0.97%
So the partial pressures of air are:
PPN2 11.47 psi or .8 atm
PPO2 3.07 psi or .2 atm
PPtr 0.16 psi
For scuba, we generally skip the trace partial pressures, and round the PPN2/O2 to 80% and 20%. The trace gas amounts do become important when gas blending using air for trimix when anoxic mixes are used for extreme depth. An atmosphere (atm) is 14.7 psi.
As we dive, we have to breathe air at ambiant pressure or we can't breathe at all! As the pressure increases, the partial pressures also increase.
This is where Henry's Law comes in.
As the ambient pressure and partial pressures increase, our body is still saturated with gas at surface partial pressures. When the ambient pressure increases, it will slowly drive gas into solution in our body, as the body is at a vacuum compared to ambient pressure. This is how our bodies become saturated with nitrogen when we dive.
Time also becomes important. The length of time we are exposed to high partial pressures determines the amount of saturation in our tissues. It takes 24 hours to become completely saturated, and also the same for desaturation.
Our bodies can withstand a 2:1 pressure differental of nitrogen before bubbles of serious nature begin to form. In terms of PPN2 this is 1.6 atm/.8 atm. Microbubbles begin to form during ascent, and as long as they stay microbubbles there is no harm. It is when they begin to come together and form larger bubbles that gives us decompression sickness (DCS). J. S. Haldane studied this problem with cassion workers, and developed the first staged decompression tables. These tables are the basis of the US Navy Diving Tables, and other decompression tables. He found that if we slowly decompress, we keep the gas from forming bubbles.
It is the same with shaken bottles of soda. Pop the top quickly, and you get a bath! Release the pressure slow, and you stay dry.
If we keep our bodies within the 2:1 differental, we can surface with generally no ill effects. This is the basis for the No Decompression Tables. With these tables, we are allowed to stay at depth only a limited amount of time. If we stay longer, we will have to go through decompression before surfacing. Below is the US Navy No Decompression Table.
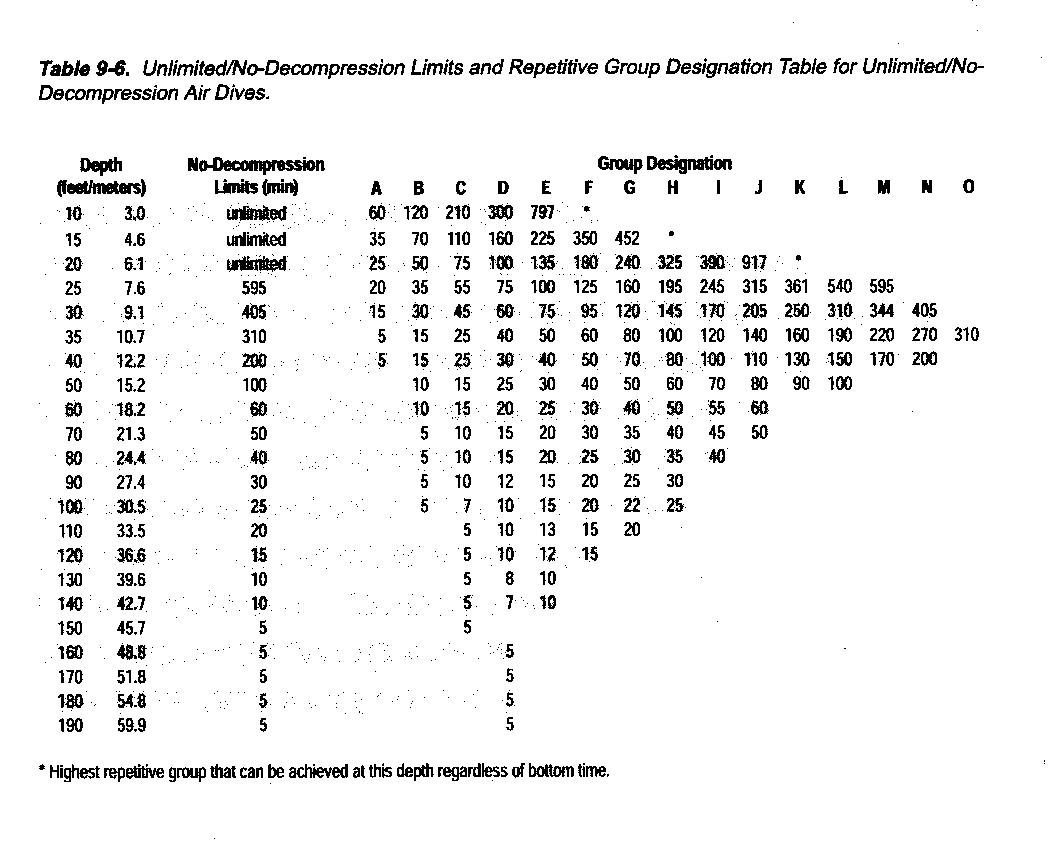
With this table we can see the limits placed on us that allow us direct access to the surface. In the first 20 feet, we can go into total saturation and still go directly to the surface. Any deeper than 20 feet, and saturation will mean required decompression. As we can see, dive time falls off the deeper we go. With the NDT, there are still factors that it cannot account for, and caution about pushing those limits is advised. For military purposes, the above table is considered correct at a 92% sucess rate. For normal recreational diving, 100 feet is considered the maximum depth without additional training.
When we go into decompression the PPN2 is at 1.6 atm. On our way back to the surface, we must stop and let the PPN2 in our bodies fall below 1.6, before proceeding again. Depending on the amount of saturation, stops may need to begin at 60 feet or deeper, and stops every 10 feet are required all the way to the surface. After we surface, it will take another 24 hours to completely desaturate. Flying in aircraft during this time is prohibited, as the reduction in pressure may raise the body over the 2:1 mark, and DCS occurs.
Nitrox
Nitrox is a mixed gas also called enriched air. Nitrox blends may go up to 50% oxygen. The lower partial pressures of nitrogen allow more time at a given depth. However, oxygen at higher partial pressures becomes toxic, so this limits the depth at which a blend can be used. The higher the O2, the shallower the depth. Pure O2 can be toxic when it's partial pressure reaches 1.6 atm or about 20 feet in depth. 50% Nitrox and pure O2 can be used as decompression gasses, as they have reduced PPN2, which allows a more rapid N2 offgassing rate. With nitrox, both nitrogen and oxygen exposure must be tracked.
Trimix
Trimix is similar to Nitrox but with helium added to the mix. The helium offsets the narcotic effects of high PPN2, and the nitrogen offsets the effects of high PPHe. Depth is again limited by O2 toxicity. For deep dives, mixes with less than 11% O2 may be used. These hypoxic mixes must not be breathed above their minimum depth as death will occur.
Freediving
Freedivers also load with nitrogen. By Boyle's Law, the air in their lungs compresses, and with that compression, the partial pressures of both N2 and O2 rise. This over numerous dives, builds up the N2 levels in the tissues of the diver. If it goes too far, DCS may occur. DCS is being seen quite often on dives past 200 feet. Freediving after scuba is not recommended, as the body is already close to saturation, and the fast ascent times may trigger DCS.
Another problem can occur in freediving, and that's Shallow Water Blackout (SWB). While most cases of SWB are from simply pushing your times, or not resting long enough between dives, the most insideous case is caused by the body/lung PPO2 problem. At the beginning of the dive, the air in the lungs is at close to 20% and the body is about the same. During the dive the PPO2 rises in the lungs and equalizes into the bloodstream, and the reverse is true for the PPC02. During ascent from dives deeper than 30 feet, the PPO2 drops in the lung to below 11%, and causes oxygen to flow out of the body and into the lung. The body sensing the low oxygen level in the lungs shuts down to conserve oxygen, even though there may be a 14-15% oxygen level in the body, and enough to complete the dive safely. This problem is solved by slowing down the ascent and giving time for levels to adjust. SWB usually happens in the last 15 feet of the dive.
to be continued......Gotta work to eat!
Last edited by Capt Nemo; 12-19-2012 at 12:06 PM.
 Posting Permissions
Posting Permissions
- You may not post new threads
- You may not post replies
- You may not post attachments
- You may not edit your posts
-
Forum Rules




 Reply With Quote
Reply With Quote
Bookmarks